Turning Point for Nuclear Medicine!
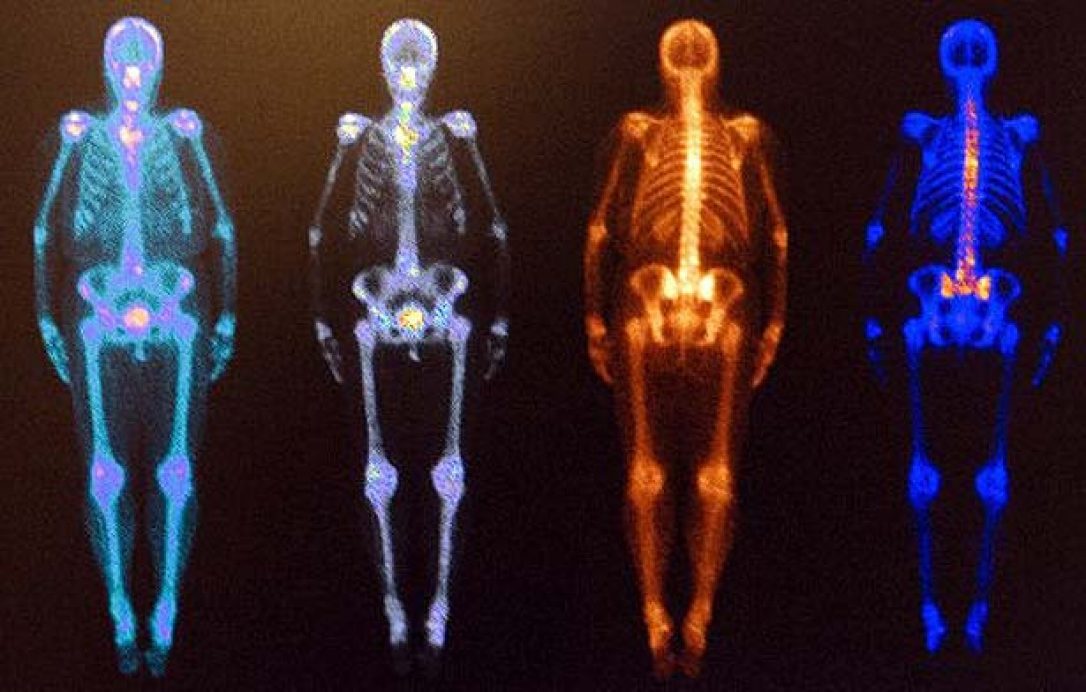
The U.S. Food and Drug Administration (FDA) announced Thursday, Feb. 7, it has approved domestic production of technetium-99m (Tc-99m), the most commonly used radioactive imaging isotopes.
Until the approval, the imaging tracer was only available from foreign suppliers, often limiting supplies of Tc-99m, which, according to the FDA, is used in more than 80 percent of nuclear imaging procedures in the U.S., along with its parent element molybdenum-99.
“Every day, tens of thousands of people in the U.S. undergo a nuclear medical imaging procedure that depends on Tc-99m,” said Janet Woodcock, MD, and director of the FDA’s Center for Drug Evaluation and Research in the release. “This radioisotope is vital to disease detection, yet health care professionals have faced challenges with adequate supply due to a complex supply chain that sometimes resulted in shortages. Today’s approval has been the result of years of coordination across the FDA and with U.S. government organizations and marks the first domestic supply of Mo-99—the source of Tc-99m—in 30 years, which will help to ensure more reliable, clean and secure access to this important imaging agent used in nuclear medicine.”
As part of Thursday’s action, the Nuclear Regulatory Commission (NRC) is issuing guidance for nuclear pharmacies on the license amendments they must obtain in order to buy and use the RadioGenix System. The NRC is the government agency responsible for regulating production and possession of radioactive materials.





Leave A Comment